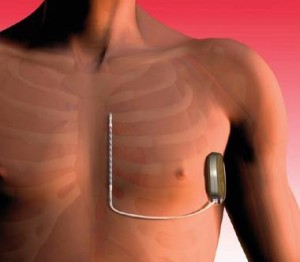
The use of ICD’s is an established therapy for the prevention of death from ventricular arrhythmia. Recently a new subcutaneous ICD (S-ICD) has been introduced, eliminating the need for transvenous lead placement in or on the heart which is mandatory in the transvenous ICD (TV-ICD). The new S-ICD therapy already proved to be feasible and safe and is an accepted therapy in Europe. It is likely that the eliminated need for transvenous lead placement substantially reduces the implantation related complications and inappropriate shock therapies and elongates lead longevity. On the other hand it is unclear whether the lack of capability to provide antitachy-pacing (ATP) in the
S-ICD may be a limitation for patients with monomorphic ventricular tachycardia. This prospective randomized controlled trial will outline the advantages and disadvantages of the S-ICD.
Objective
The primary objective of this randomized trial is to outline the advantages and disadvantages of the S-ICD. We will study whether the S-ICD is non-inferior to the TV-ICD with respect to major ICD-related adverse events (i.e. inappropriate shocks, acute and chronic implant related complications and lead- or device related complications). If non-inferiority is established, superiority of the S-ICD as compared to the TV-ICD will be tested.